Abstract
Objective: To evaluate the efficacy and safety of decitabine combined with IA regimen in the treatment of newly diagnosed acute myeloid leukemia.
Methods: From September 1, 2013 to October 18, 2019, 164 newly diagnosed acute myeloid leukemia patients who received IA or decitabine combined with IA induction chemotherapy who were hospitalized in the Department of Hematology of the First Affiliated Hospital of Sun Yat-sen University were enrolled. The efficacy and side effects of treatment were analyzed.
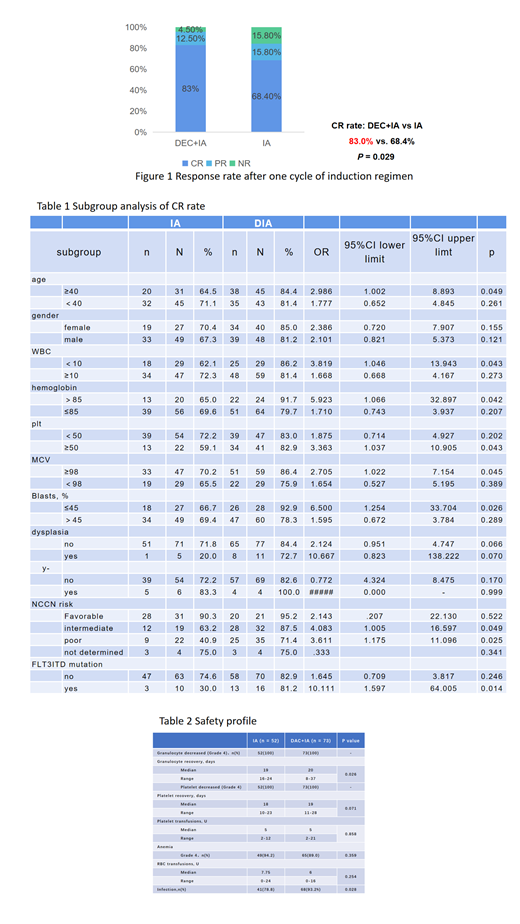
Results: The complete remission rate of decitabine combined with IA regimen chemotherapy group (n=88) and IA regimen chemotherapy group (n=76) was 83.0% vs. 68.4% (P=0.029, Fig 1). Subgroup analysis (table 1) showed that age ≥40 years old, WBC<10*10^9/L, Hb>85g/L, PLT≥50*10^9/L, MCV≥98fL, ratio of bone marrow immature cells ≤45%, NCCN intermediate-risk or high-risk group, patients with FLT3ITD mutation had a higher CR rate in the decitabine combined with IA regimen group. Multivariate analysis showed that combined decitabine was an independent favorable factor affecting the CR rate (OR 3.559, 95% CI: 1.554-8.151, P=0.003). Compared with the IA group, patients in the decitabine combined with IA group took longer to rebuild the granule system (20 days vs 19 days, P=0.026), and the incidence of infection was higher (93.2% vs 78.8%, P=0.028) (table 2).
Conclusion: Compared with the IA regimen, the decitabine combined with the IA regimen significantly improves the induction chemotherapy response rate of newly diagnosed non-M3 AML patients, especially for patients with the following characteristics: age ≥ 40 years old, WBC <10*10^ 9/L, Hb>85g/L, PLT≥50*10^9/L, MCV≥98fL, bone marrow immature cell proportion ≤45%, NCCN risk stratification medium-risk or high-risk group, FLT3ITD mutation. After combining with decitabine, the patient's granular bone marrow suppression increased.
No relevant conflicts of interest to declare.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract